Count the total number of valence electrons in the structure Remember Group of Valence electrons 2. As a result central carbon in the CCl4 Lewis Structure with all four Chlorines arranged around the tetrahedral geometry.
Draw the Lewis structure for the SeH2 molecule.
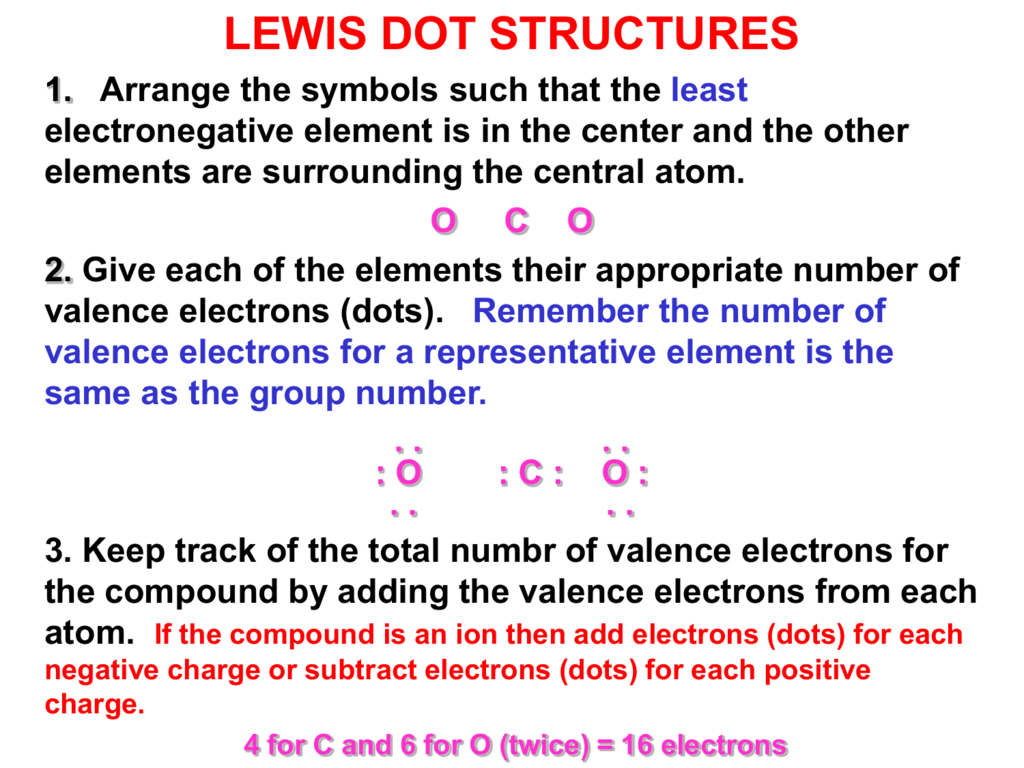
. Regarding this how do you find the central atom when drawing a Lewis structure. Had to come up with my own. Draw the Lewis structure for the Se and 2 H atoms.
Chemistry questions and answers. Remember the trend for electronegativity on the periodic table. Hydrogen is never the central atom.
MUST FOLLOW IN ORDER. The central atom is also usually the least electronegative. 22 Draw the best Lewis structure for Cl3.
Key Points To Consider When Drawing The O2 Electron Dot Structure. Learn Exam Concepts on Embibe In Lewis Structures a line is used to represent the bonding electrons between two combining atoms. Decide on a skeletal structure What is bonded to what The central atom is generally written first in the formula.
Decide which atom is the central atom in the structure. Water molecule is a simple molecule. If an atom occurs only once this atom is most likely to be the central atom exception is.
Hydrogen atoms are joint to oxygen atom through single bonds. The atom with the least electronegative charge should. I gave up on Lewis structure.
Answer 1 of 3. Determine the total number of electrons available for bonding. Metals will almost always be the central atom if they are present.
Now as we continue to review how to draw Lewis structures we must decide which atom will be drawn in the center of our Lewis structure. Draw the Lewis structure for the SeH2 molecule. A If there are more than one of the least electronegative atom your skeletal structure should have those two attached to one another EXCEPTION.
Compared with ionic compounds molecular compounds a. In drawing Lewis structures for relatively small molecules and polyatomic ions the structures tend to be more stable when they are compact and symmetrical rather than extended chains of atoms. Draw a Skeletal Structure.
A-1 B 0 C 1 D 2 E-2 23 Draw the best Lewis structure for the free radical NO2. H2O there are two H and only one O so O is the central atom even though it is the most electronegative atom. The order of electronegativity for the nonmetals is FONClBrISCH.
Here are the steps that I use to draw a Lewis structure. KrF2 or Krypton difluoride is made up of Krypton and Fluorine and is one the first compounds of Krypton. Find the Number of Electrons Needed to Make the Atoms Happy.
Best Answer Copy The central atom in a Lewis structure is usually the least electronegative atom. Also there are two lone pairs on oxygen atom. Find the Total Number of Valence Electrons.
How do you draw Lewis structures. A three-step approach for drawing the O2 Lewis structure can be used. Drawing lewis structure of water molecule is simple than some of other complex molecules or ions.
The central atom in a Lewis structure is usually the least electronegative atomEDITIf an atom occurs only once this atom is most likely to be the central atom exception is hydrogen as it can. Once determined draw that element by atomic symbol in the center and draw single bonds to the other atoms. Atom with the greatest mass c.
The central atom is the least most electronegative atom in the compound. In simple compounds the central atom is the element that appears only once in the formula example. Atom with the fewest electrons b.
If all of the atoms usually form the same number of bonds the least electronegative atom is. H 2 O lewis structure. How many electron domains are around the central atom.
Choose a Central Atom. Put a box around the lewis structure with a charge. In drawing a Lewis structure the central atom is the a.
Draw the Lewis dot structure for eqXeI_4 eq and answer the following questions. The primary exception to this rule is where H is bonded to more than one other atom. Have higher boiling points c.
Place Remaining Electrons Around the Central Atom. 1 Instead I think of electron configuration and the exceptions by. Count the valence electrons in your trial structure.
Write the Lewis structure for CH2O where carbon is the central atom. H and F are almost always terminal. What is the central atom in a Lewis structure.
Usually the central atom will be the one that has the most unpaired valence electrons. Atom with the highest atomic number d. Place Electrons Around Outside Atoms.
The first step is to sketch the Lewis structure of the O2 molecule to add valence electrons around the two oxygen atoms and the final step is to combine the two oxygen diatomic atoms to get the O2 Lewis Structure. When drawing a lewis structure of polyatomics what do you do that differentiates it from other lewis structures because it. Only full subshells Subshells are longitudinal rings in two hemispheres A separate transition equatorial subshells -eq of 3 to handle.
First draw the Lewis dot structure. Draw a skeleton structure in which the other atoms are single-bonded to the central atom. In a Lewis Structure electrons are represented as dots surrounding the central metal atom.
The central metal is denoted by using its chemical symbol from the Periodic Table. Determine which atom will be the central atom of the Lewis Dot Structure. The central atom of a molecule is usually the least electronegative atom or the atom with the highest valence.
The central atom is usually the atom with the lowest subscript in the molecular formula and the atom that can form the most bonds. Lewis Structures Rules for Drawing Lewis Structure. Draw the Lewis structure Step 2.
What is the formal charge on the central Cl atom. We have found the total number of valence electrons. To find electronegativity either rely on periodic table trends or consult a table that lists electronegativity values.
What is the formal charge on the N. Calculate the total number of valence electrons in the molecule by adding the valence electrons from each atom. Draw a trial structure by putting electron pairs around every atom until each gets an octet.
The central atom in a lewis structure is always what as long as it is not hydrogen. In the lewis structure of H 2 O there are two single bonds around oxygen atom. Combining step1 and step2 to get step3 for C.
In a CCl4 Lewis Structure diagram the carbon atom can be the centre atom.
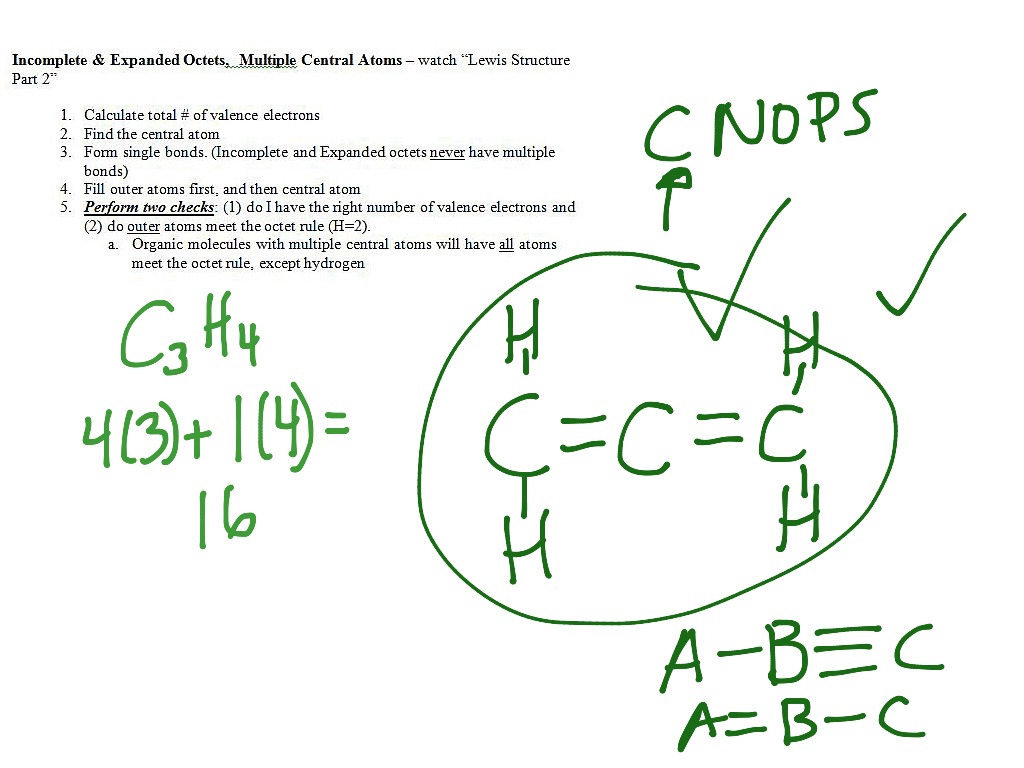
Lewis Structures Multiple Central Atoms Science Chemistry Chemical Bonds Showme

Lewis Structures Chapter 8 Lewis Structures Lewis Structures Are Representations Of Molecules Showing All Valence Electrons Bonding And Nonbonding Ppt Download
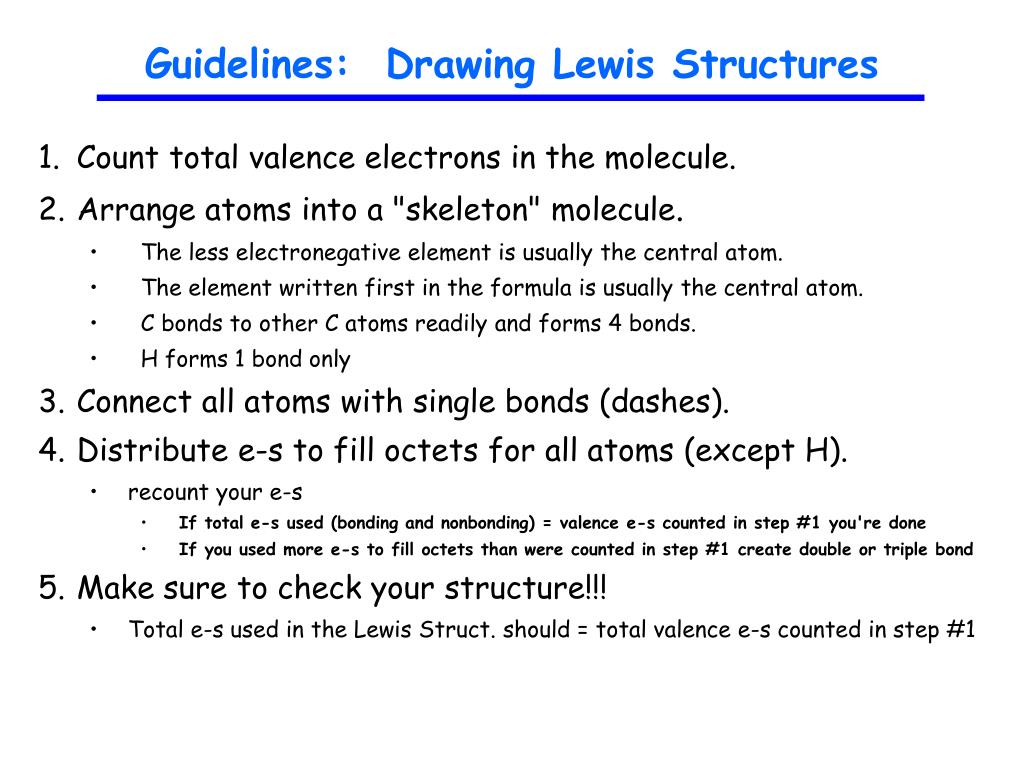
Ppt Guidelines Drawing Lewis Structures Powerpoint Presentation Free Download Id 1812949

Ppt Drawing Lewis Structures Powerpoint Presentation Free Download Id 529421

Step 1 Identify The Central Atom And Draw It S Lewis Structure This Is The Element With The Lowest Number Of Atoms In The Formula Draw A Lewis Dot Diagram Ppt Download

How To Draw Lewis Structures For A Molecule With One Central Atom No Octet Rule Exceptions Chemistry Study Com

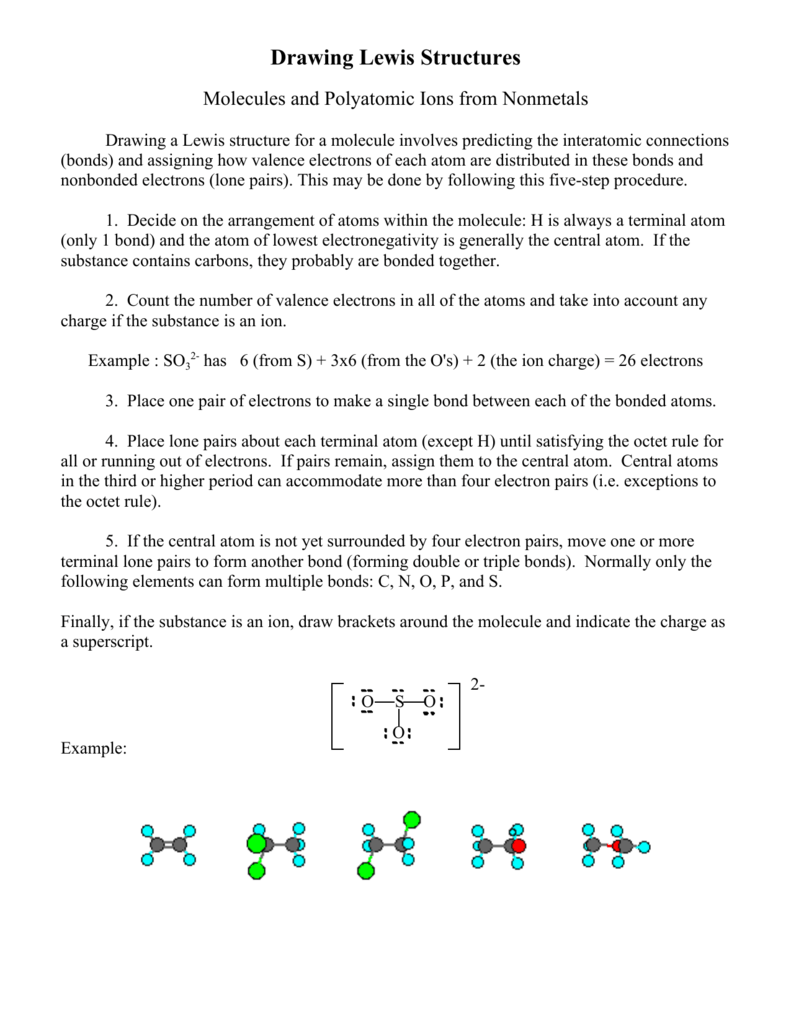
0 comments
Post a Comment